Source from Bloomberg Terminal.

• SVB Leerink and Roche warn that don’t be too positive about the launching time of the vaccine.
• The analyst indicates that rushing the vaccine development may bring risks.
The bell tolls. The rebounding US stock seems to be too positive toward the possibility of the launch of the COVID-19 vaccine in the short term.
A scientist of Oxford University told to The Times earlier this month that the COVID-19 vaccine will be ready in September, which is one year earlier than the estimation by the US organization.
“The comments about the vaccine and the launching time is impossible to avoid. This kind of conclusion can mislead the policymakers, investors, and developers when they expect and determine,” said Geoffrey Porges from SVB Leerink in his research report.
High expectation boosts the stock of the vaccine developing companies. Since the later February, Moderna Inc. a US pharmacy company, and other companies in a smaller size, Novartis, Inovio, and Pharmaceuticals have risen double time in their stock price, although these companies all failed to launch any product to the market before the pandemic broke out. BioNTech SE who cooperates with Pfizer increased significantly on April 29th. Before this, there was news that the company was going to begin the COVID-19 vaccine test in Germany.
“Really Progressive”
Porges is not the only buzzkill. CEO of Roche Severin Schwan said through the Bloomberg TV on Wednesday that developing a vaccine usually takes a couple of years. Spending only 12-18 months seems really progressive.
Associate Professor of Pharmacy at Harvard Medical School, Director of the Vaccine and Immunotherapy Center of Massachusetts General Hospital, Mark Poznansky said that the investors think Moderna will be the fastest one (to develop the vaccine), but we have to wait twelve more months to ensure whether the mRNA of the company is safe and effective.
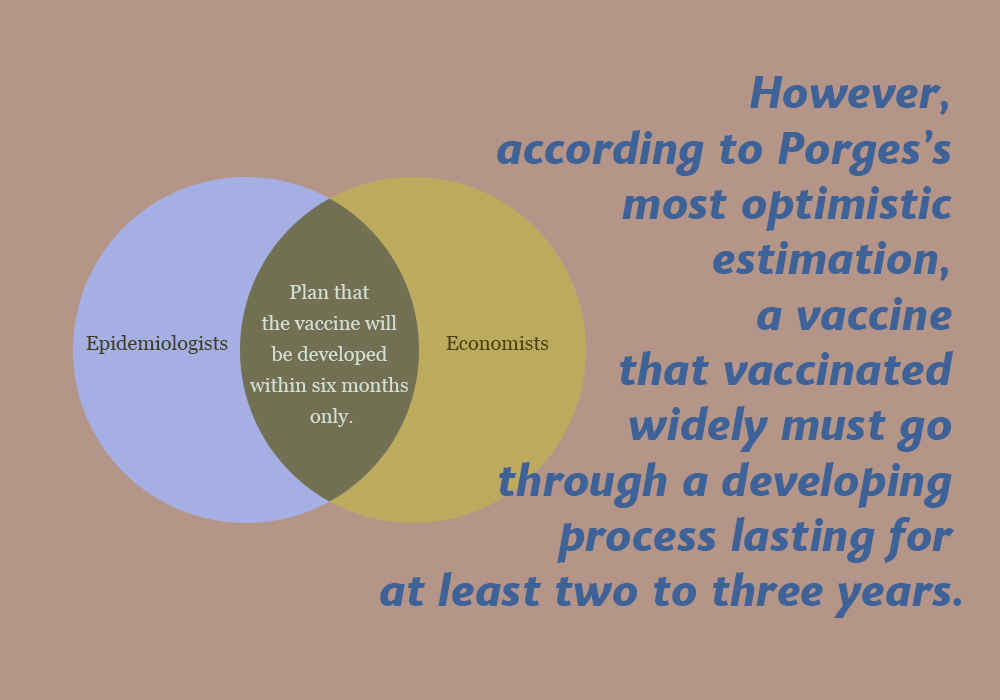
As Porges said, the problem is that epidemiologists and economists are seemed to plan that the vaccine will be developed within six months only. However, according to Porges’s most optimistic estimation, a vaccine that is vaccinated widely must go through a developing process lasting for at least two to three years.
Porges owns not only the MBA degree of HBS but also the Bachelor of Pharmacy at the University of Sydney. Before he came to Wall Street, he worked for Merck, responsible for the commercial strategy, product planning, and commercialization of Merck vaccine products.
Porges said that although some vaccines are set at a faster-developing speed, such as the adenovirus-based vaccine by Moderna or Johnson & Johnson, too fast may risk safety or efficacy.
